
Company news
Lixin Pharmaceutical's nilotinib hydrochloride API was approved as "A" in China
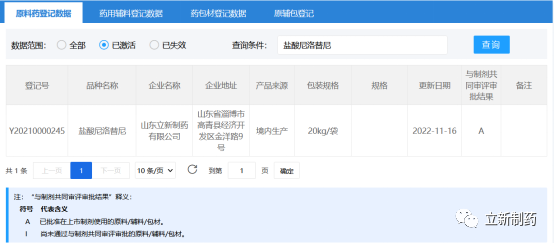
According to the official website of the State Drug Administration on November 16, Shandong Lixin Pharmaceutical Co., Ltd.’s nilotinib hydrochloride raw material drug [registration number: Y20210000245] has passed the technical review of the Center for Drug Evaluation (CDE), becoming the first drug manufacturer in China The company whose registration platform of CDE's raw and auxiliary package has been approved to be transferred to A is also the first global generic of the API to be officially recognized by CDE.
Nilotinib hydrochloride is a highly selective tyrosine kinase inhibitor antineoplastic drug, which is used to effectively treat drug-resistant or intolerant chronic myeloid leukemia patients.
The synthesis process of nilotinib hydrochloride is complex and requires high quality. The R&D team of Lixin Pharmaceutical has concentrated its superior resources, overcome difficulties along the way, successfully broke through a number of technical barriers, and formed technical achievements with distinctive characteristics and competitive advantages in many aspects such as green technology, impurity research, and crystal particle size. This "first imitation" will surely become an important milestone in the company's development history.
In addition, Lixin Pharmaceutical has extensively deployed nilotinib hydrochloride API products in the global market. At present, nilotinib hydrochloride registered by Suzhou Lixin Pharmaceutical Co., Ltd. in the US FDA has passed the DMF technical review, which is sufficient to support the listing of the preparation ANDA. At the same time, Suzhou Lixin and Shandong Lixin have been registered in Canada and EU EDQM respectively, and both have entered the review and approval stage, and will soon be approved for listing.
Lixin Pharmaceutical has been adhering to the implementation of global norms, strengthening international cooperation, international registration, and international certification, and has passed the certification of FDA, EDQM, EMA, PMDA and other authoritative organizations for many times. The company will continue to increase international certification and registration efforts, give full play to its international advantages, and expand the international market.
Related New
2024.01.16
2024.01.16
2023.03.21
2022.10.20
2022.08.31