
Company news
Lixin Pharmaceutical's hydrochlorothiazide was approved again as a double "A"
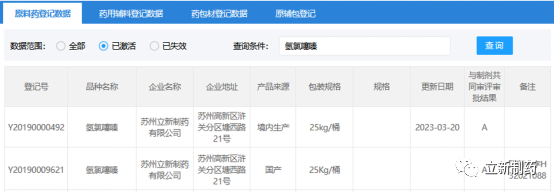
According to the official website of the State Drug Administration on March 20, Suzhou Lixin Pharmaceutical Co., Ltd.’s hydrochlorothiazide raw material drug [registration number: Y20190000492] has passed the technical review of the Center for Drug Evaluation (CDE), and has been publicized on the registration platform of CDE as "A" became the first company to be newly registered and approved after the implementation of the registration and filing system for domestic APIs, which also made Lixin Pharmaceutical a double "A" enterprise with this product.
Hydrochlorothiazide is a commonly used diuretic and antihypertensive drug. As a traditional superior variety of Suzhou Lixin Pharmaceutical, this variety was approved by the State Administration of Medicine in 2002 [Guoyao Zhunzi: H32021088], and was successfully registered and transferred to "A" according to the approval number of raw materials [registration number: Y20190009621]. At the same time, in order to expand the international market, Lixin Pharmaceutical has completed FDA registration in 2007, and obtained the CEP certificate of the European Union EDQM in 2014. Through high-quality products and services, it meet the preparation production needs of many domestic and foreign preparation customers.
With the continuous expansion of the application of hydrochlorothiazide in the field of cardiovascular disease treatment, the quality requirements for its raw materials are increasing day by day. On the basis of the original hydrochlorothiazide process, Lixin Pharmaceutical continues to cultivate and expand, and overcome difficulties along the way. In environmental protection, crystallization technology and impurity control Breaking through a number of technical barriers, the "new version" of hydrochlorothiazide, which has stricter quality standards than EP&USP standards, and conforms to ICH Q3D&ICH M7 for elements and genotoxic impurities, meets the individual needs of different customers in the consistency evaluation. Quality and safety provide premium guarantee.
Lixin Pharmaceutical will provide customers with a full range of competitive and differentiated products with the advantages of world-leading quality, green technology and efficient and professional services, benefiting more patients and a wider pharmaceutical market.
Related New
2024.01.16
2024.01.16
2022.11.17
2022.10.20
2022.08.31