
Company news
Lixin Pharmaceutical became the first company in the world to have the CEP certificate of Peimei [2.5 water and 7 water] at the same time

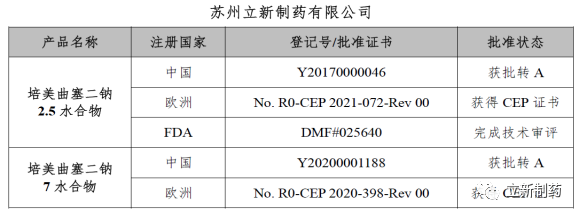
Recently, Suzhou Lixin Pharmaceutical Co., Ltd. received the CEP certificate for Pemetrexed Disodium Hemipentahydrate issued by the European Agency for the Quality of Medicines (EDQM), plus the approved Pemetrexed Disodium 7 Water (Pemetrexed Disodium Heptahydrate) CEP certificate, making Suzhou Lixin Pharmaceutical Co., Ltd. the first company in the world to have both Pemetrexed Disodium 2.5 water and 7 water CEP certificates. The issuance of this certificate shows that the product quality and R&D technical capabilities of the API have obtained the approval and sales qualification of the European standard market, which will help the company expand the international high-end market of the product and enhance the company's image and brand in the entire industry chain of API has a pivotal role.
Name of raw material: Pemetrexed disodium 2.5 water
Certificate number: No. R0-CEP 2021-072-Rev 00
Expiration date: From July 8, 2022, the validity period is 5 years
Pemetrexed disodium is a drug containing Antifolate preparations with pyrropyrimidine structure can inhibit the growth of tumors by destroying the normal metabolic process dependent on folic acid in cells and inhibiting cell replication.
Pemetrexed disodium, as a key and advantageous product of Lixin Pharmaceutical, is one of the anti-tumor products that the company has deeply cultivated and extensively deployed. Peimei 2.5 water has been approved for marketing in China and Europe, and the FDA has completed the technical review; Peimei 7 water has been approved for marketing in China and Europe. The quality standards of our pemetrexed disodium series products are stricter than EP standards and USP standards. The elemental impurities and genotoxic impurities all meet the requirements of ICH Q3D and ICH M7, meet the registration requirements of domestic and foreign laws and regulations, and provide high-quality products for injection products.
Lixin Pharmaceutical has been adhering to the implementation of global norms, strengthening international cooperation, international registration, and international certification, and has passed the certification of FDA, EDQM, EMA, PMDA and other authoritative organizations for many times. The company will continue to increase international certification and registration efforts, give full play to its international advantages, and expand the international market.
Related New
2024.01.16
2024.01.16
2023.03.21
2022.11.17
2022.10.20